Development of a CO2 Direct Ocean Capture System Using Bipolar Membrane Electrodialysis
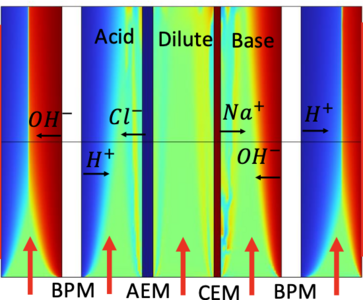
A CO2 direct ocean capture system exploiting bipolar membrane (BPM) electrodialysis, gas-liquid contacter and pH-swing of the dissolved inorganic carbon species is designed for efficient and effective CO2 capture. COMSOL Multiphysics was used to study the BPM electrodialysis and CO2 ocean drawdown process involved in the system and optimize the system. (1) BPMs are membranes comprised of an anion-exchange layer and a cation-exchange layer. When potential is applied, BPM can promote water dissociation and therefore generate acid and base. A 2D model that combined electrochemistry, fluid dynamics and mass transport was developed to study the process of acid and base generation in the BPM electrodialysis cell. The model was used to optimize the geometry of the electrodialysis cell for better efficiency and durability. (2) A 3D model was developed to study the turbulent mixing and reactions in the CO2 drawdown process. During the process, the decarbonized, alkalinized water is discharged into the ocean to promote CO2 drawdown. The model considers the turbulence enhanced mixing of the chemical species and the buffering kinetics of the reactions between dissolved CO2, carbonate and bicarbonate. Based on simulation results, the impact of the discharge on the local chemical environment and the drawdown rate is evaluated. The model predicted that the pH of the plume restores to a value close to natural ocean within a very small distance while the enhanced CO2 drawdown continues to happen at a larger length scale.