Developing a Growth Prediction Model of Abdominal Aortic Aneurysms via Computational Fluid Dynamics
Veröffentlicht in 2024
Abdominal Aortic Aneurysms (AAAs) are balloonings of the descending aorta. Surgical intervention only occurs when the aneurysm growth exceeds a certain threshold, which is slightly different between men and women. Early-stage patients are often asymptomatic, and there is no prediction model to estimate how the aneurysm would progress in a patient. Clinically, the maximum diameter of the aorta is closely monitored to assess the growth of AAA. Previous studies have shown that other parameters, including curvature and torsion, could offer more insight into aneurysm growth. However, these geometric studies fail to analyze blood flow parameters that can significantly influence aneurysm growth. Within AAAs, thrombus often develops to strengthen the vessel wall, and it is oftentimes calcified. While a calcified thrombus could serve as a potential solution to support the weakened aortic wall, it has been shown to cause other problems, such as increased local shear stress, which can increase the risk of aneurysm rupture. The goal of this study was to develop computational fluid dynamics models to evaluate how blood flow-induced stress affects the growth rate of AAAs.
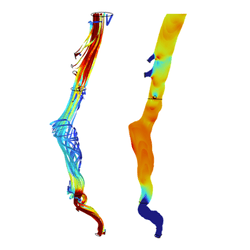
Three-dimensional geometries of abdominal aortic aneurysms were established via segmentation of CT-scans of six patients using ITK-SNAP. In COMSOL Multiphysics, a laminar model was developed to simulate blood flow conditions within the patients’ abdominal aorta. Blood was modeled as a Newtonian flow with a density of 1,060 kg/m3 and a dynamic viscosity of 0.0035 Pa·s. The celiac, mesenteric, renal, and iliac arteries were defined as pressure outlets. The results demonstrated an increase in average surface pressure over time in fast growing aneurysms, yet decreased in slow growing aneurysms. Average shear stress appeared to have a large increase over time in fast-growing aneurysms. However, no significant results between the slow or fast growing aneurysms were noted.
In the next phase of this project, we plan to develop a Fluid-Structure Interaction (FSI) model of the AAA, which will include four domains: the blood volume, thrombus, calcification, and the arterial wall. Maximum deformable pressure is planned to be used for this model. All four domains will be fully coupled. By gathering the velocity, shear stress, and surface pressure from this next phase investigation, we aim to identify key mechanical parameters that correlate with the growth rate of the aneurysm, which may serve as the bases to create a predictive model, providing a better way to prepare treatment for patients with AAAs.
We would like to thank Dr. Apostolos Tassiopoulos’s group in the Department of Vascular Surgery at Stony Brook University Hospital for providing the aortic aneurysm CT scans, segmentation masks, and guidance on segmentation. We would also like to thank Dr. Marina Fandaros for aiding in the development of the modeling procedure.