Deep Neural Network Surrogate Model for Blood Damage Modeling in FDA Hemolysis Benchmark
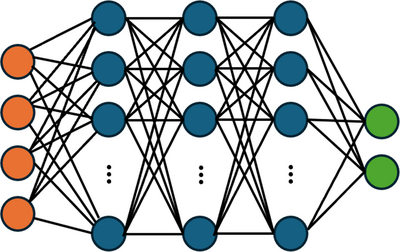
A key challenge in the design of medical devices that interface with blood is to minimize the likelihood of blood damage. Simulating blood flow through a medical device and estimating the likelihood of blood damage is a key step to improving performance and safety, thereby expediting device development and regulatory approval. In this presentation, we describe how to train and deploy surrogate models to provide real-time visualizations of blood damage and the effects and tradeoffs associated with changes to design and operating parameters. Such real-time visualizations are extremely useful to facilitating design discussions, but are generally not possible with traditional FEA simulations due to their high computational cost. Accurately predicting blood damage is also challenging, since it accumulates over time and occurs incrementally when blood cells venture near walls or high-shear-stress regions of the flow. Herein, we define “blood damage” as hemolysis measured by an increase in non-bound hemoglobin in the blood circulating through a device. This quantity is readily measured in experiments, although other considerations such as sublethal damage and thrombosis are important as well. Our surrogate model comprises a Deep Neural Network (DNN), introduced in COMSOL Multiphysics® 6.2, trained on our past “full model” simulations [1] of blood damage in the FDA benchmark nozzle [2] (Figure 1). Our full model uses a stress-based power law hemolysis model to predict changes in hemoglobin (Figure 2). We review effective practices for surrogate model training and for choosing the structure of the deep neural network and its activation functions. Though the quantities of interest (e.g., shear stress, rate of hemolysis) contain sharp local gradients, the experimentally measurable endpoint quantity (cumulative hemolysis) is smoothly varying and thus trainable for a surrogate model. To validate our full and surrogate models, we compare their predicted hemoglobin concentrations with the experiments of Herbertson et al. [3] While the present work focuses on the publicly available FDA benchmark geometry [2], our methodology may be used to estimate blood damage in a wide variety of device features including instrumentation loops and device-blood vessel connections.