Updates im Electrodeposition Module
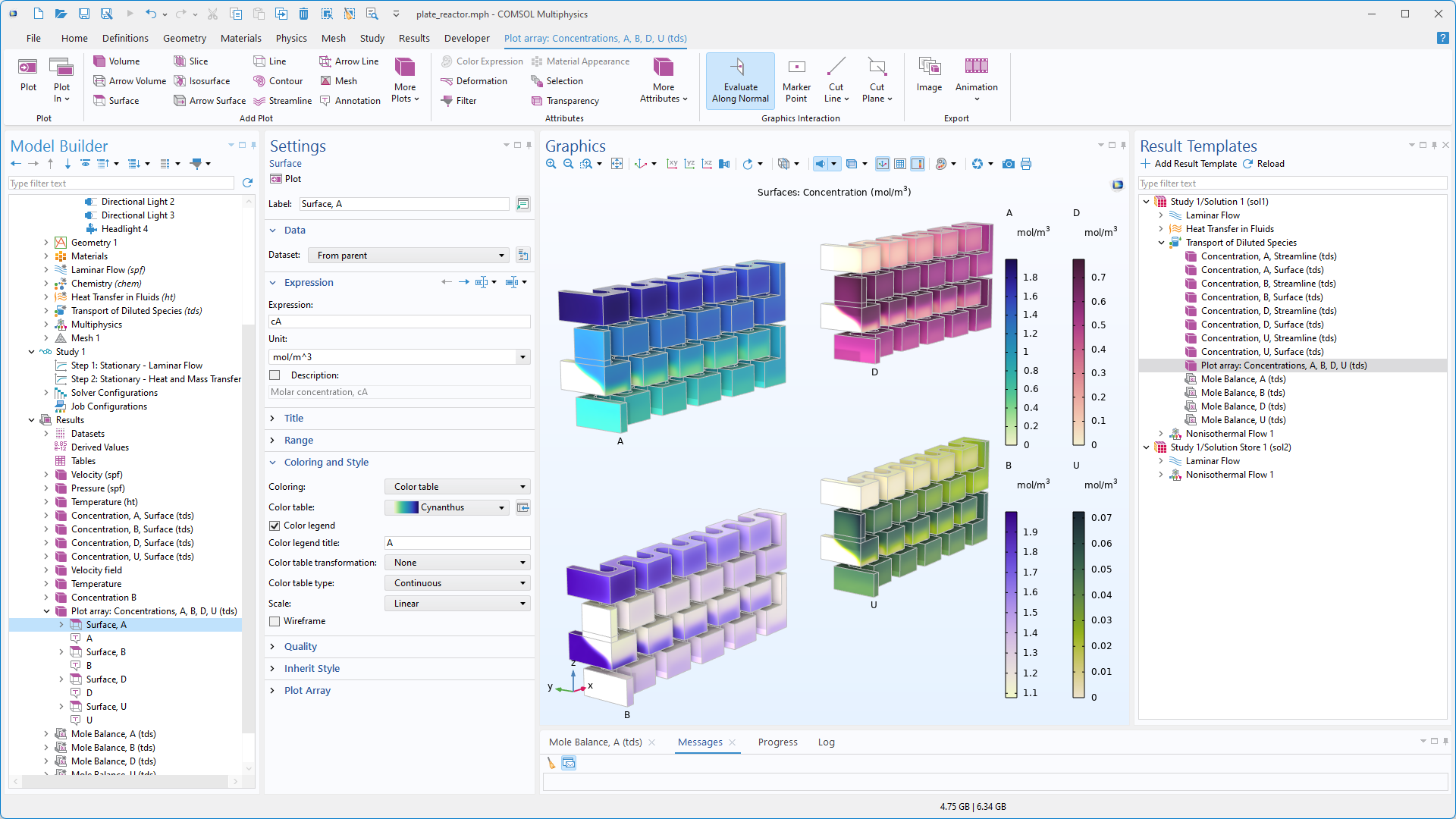
Interface Concentrated Electrolyte Transport
Ein Interface Concentrated Electrolyte Transport ist jetzt verfügbar, um den Transport in jeder beliebigen Elektrolytlösung mit einer beliebigen Anzahl geladener und ungeladener Spezies zu modellieren. Dieses elektrochemische Interface basiert auf der Theorie konzentrierter Lösungen, bei der die Transportgleichungen unter Verwendung binärer Maxwell-Stefan-Diffusionskoeffizienten definiert werden, wobei lokale Elektroneutralität vorausgesetzt wird. Im Gegensatz zu den Nernst-Planck-Gleichungen geht die Theorie konzentrierter Lösungen nicht davon aus, dass die Elektrolytspezies in einem neutralen Lösungsmittel konstanter Konzentration verdünnt sind. Typische Elektrolyte, die modelliert werden können, sind ionische Flüssigkeiten, Salzschmelzen und hochkonzentrierte Lösungen mit nicht vernachlässigbaren Konzentrationsgradienten der ladungstragenden Spezies. Das neue Tutorial-Modell Molten Carbonate Transport veranschaulicht diese Funktionalität.
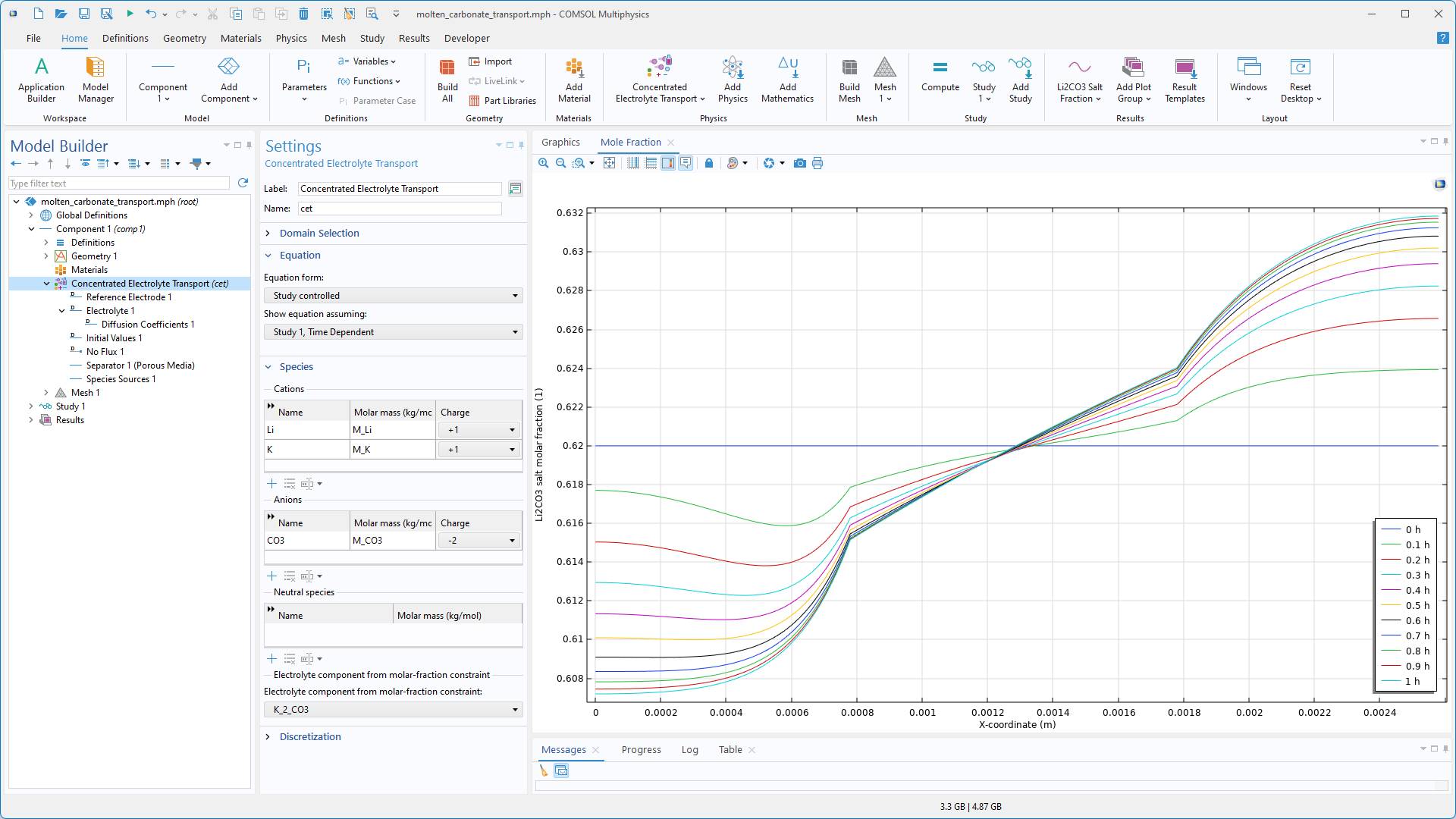
Parameterschätzung
Der Studienschritt Parameter Estimation und die Optimierungslöser BOBYQA, Levenberg–Marquardt und IPOPT sind jetzt im Electrodeposition Module verfügbar. Die Parameterschätzung wird zur Bestimmung von Modellparametern durch Anpassung von Simulationen an experimentelle Daten verwendet.
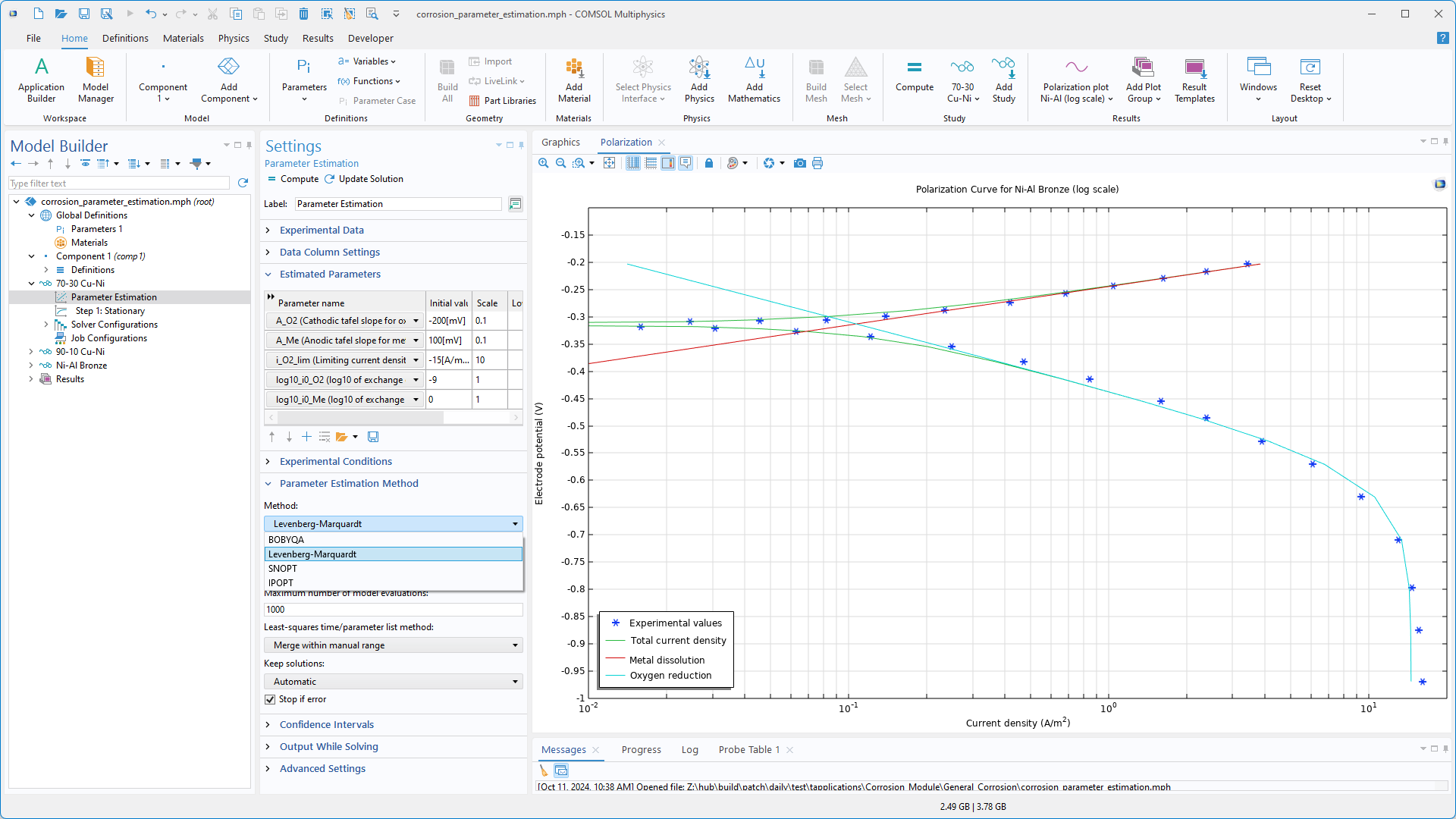
Result Templates in den Interfaces Chemical Species Transport
Das Erstellen nützlicher und visuell ansprechender Plots von reaktiven Systemen kann zeitaufwendig sein, da oft viele Reaktanten und somit viele Konzentrationsfelder zu plotten sind. Um Zeit zu sparen, gibt es eine Reihe neuer Result Templates in den Interfaces Chemical Species Transport. Darunter sind jetzt Plot-Array-Vorlagen verfügbar, die bis zu vier Spezieskonzentrationen gleichzeitig im Grafikfenster anzeigen. Die Result Templates sind für alle Interfaces Chemical Species Transport verfügbar, unabhängig vom Add-On-Produkt, aber besonders nützlich für die Mehrkomponenten-Transport-Interfaces, die in den Modulen für Chemietechnik sowie im CFD Module, Porous Media Flow Module, Subsurface Flow Module und Microfluidics Module enthalten sind.