Lithium-Ion Battery Aging with Varying Solvent Composition Effects
Application ID: 140241
The liquid electrolyte in lithium-ion batteries (LIBs) typically consists of a lithium salt, such as LiPF6, dissolved in one or several solvents.
Commercial LIBs commonly employ a mix of multiple hydrocarbon-based solvents along with additional additives. In an electrolyte consisting of multiple solvents, the local electrolyte transport properties within the separator and the electrodes, such as conductivity, may depend not only on the salt concentration but also on the ratio of the different solvents.
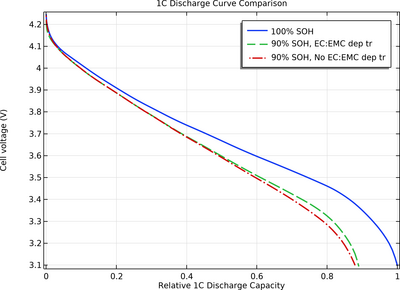
As the battery ages, parasitic reactions may predominantly consume one of the solvents, resulting in a varying ratio of the different solvents over time. This change can, in turn, alter how the electrolyte transport properties are influenced by the local salt concentration in the cell throughout the battery's lifetime.
This tutorial model explores how the consumption of one of the solvents in an aging battery cell impacts its 1C discharge capacity.
To learn more about this model, see our accompanying blog post "The Effects of Varying Solvent Compositions on Lithium-Ion Battery Aging".
Dieses Beispiel veranschaulicht Anwendungen diesen Typs, die mit den folgenden Produkten erstellt wurden:
Allerdings können zusätzliche Produkte erforderlich sein, um es vollständig zu definieren und zu modellieren. Weiterhin kann dieses Beispiel auch mit Komponenten aus den folgenden Produktkombinationen definiert und modelliert werden:
Die Kombination von COMSOL® Produkten, die für die Modellierung Ihrer Anwendung erforderlich ist, hängt von verschiedenen Faktoren ab und kann Randbedingungen, Materialeigenschaften, Physik-Interfaces und Bauteilbibliotheken umfassen. Bestimmte Funktionen können von mehreren Produkten gemeinsam genutzt werden. Um die richtige Produktkombination für Ihre Modellierungsanforderungen zu ermitteln, lesen Sie die Spezifikationstabelle und nutzen Sie eine kostenlose Evaluierungslizenz. Die COMSOL Vertriebs- und Support-Teams stehen Ihnen für alle Fragen zur Verfügung, die Sie diesbezüglich haben.
